Are Polar Molecules Able to Cross Lipid Bilayer
But still they cant enter the cell because their entry gets restricted by the presence of hydrophobic tails. Moore Justin Shorb Xavier Prat-Resina Tim Wendorff Adam Hahn.

Why Can Only Small Molecules Pass Through The Phospholipid Bilayer Of The Cell Membrane Biology Stack Exchange
Small uncharged polarmolecules such as water or urea also diffuse across a bilayer albeit much more slowly Figure 11-1.

. However small polar or ionic molecules can pass through the lipid bilayer via specific transmembrane. Ions and large polar molecules cannot pass through the. Facilitated diffusion of the glucose using a carrier protein.
By contrast lipid bilayers are highly impermeable to charged molecules ions no matter how small. Because hydrophobic nature of the lipid. These molecules pass across membranes via the action of specific transmembrane proteins which act as transporters.
Membrane proteins in a bilayer also allow for transport of ions and other molecules across the bilayer which could not cross otherwise. These molecules pass across membranes via the action of specific transmembrane proteins which act as transporters. Cotransport of the glucose with a proton or sodium ion that was pumped across the membrane using the energy of ATP hydrolysis.
Fatty acids glycerol lipid Lipid phosphate phospholipid Now consider the rule. But in the same logic hydrophobic molecules shouldnt pass through the bilayer. Movement of glucose into the cell through a glucose channel.
So the ions being polar in nature can easily cross the polar and hydrophilic head. Although ions and most polar molecules cannot diffuse across a lipid bilayer many such molecules such as glucose are able to cross cell membranes. Although ions and most polar molecules cannot diffuse across a lipid bilayer many such molecules such as glucose are able to cross cell membranes.
Yes because the lipid bilayer is polar. Charged atoms or molecules of any size are repelled by the hydrophobic tails in the interior of the phospholipid bilayer and hence cannot traverse the cell membrane by simple diffusion. Hydrocarbons O2 Co2 Polar molecules examples.
Osmosis occurs as water can cross the lipid bilayer through what. Yes Since the lipid bilayer of cells is nonpolar only non-polar substances can pass directly through the bilayer without the need for any help by membrane transport proteins. Because of the chemical and structural nature of the phospholipid bilayer hydrophobic core only lipid-soluble molecules and some small molecules are able to freely pass through the lipid bilayer.
Able to cross lipid bilayer. Passive diffusion of the glucose through the lipid bilayer. It is commonly told that hydrophobic lipophilic nonpolar molecules can quite easily pass phospholipid bilayer and hydrophilic polar or ionic molecules cant pass when no protein aid that.
Water sugars Ions examples. Such transport proteins determine the selective permeability of cell membranes and thus play a. The charge and high degree of hydration of such molecules prevents them from entering the hydrocarbonphase of the bilayer.
Polar Lipids is shared under a CC BY-NC-SA 40 license and was authored remixed andor curated by Ed Vitz John W. Large hydrophilic polar or ionic molecules cannot easily pass the phospholipid bilayer. The cell membrane is permeable to water molecules even though they are polar due to the.

How Can A Non Polar Molecule Pass Through Cell Membrane Quora
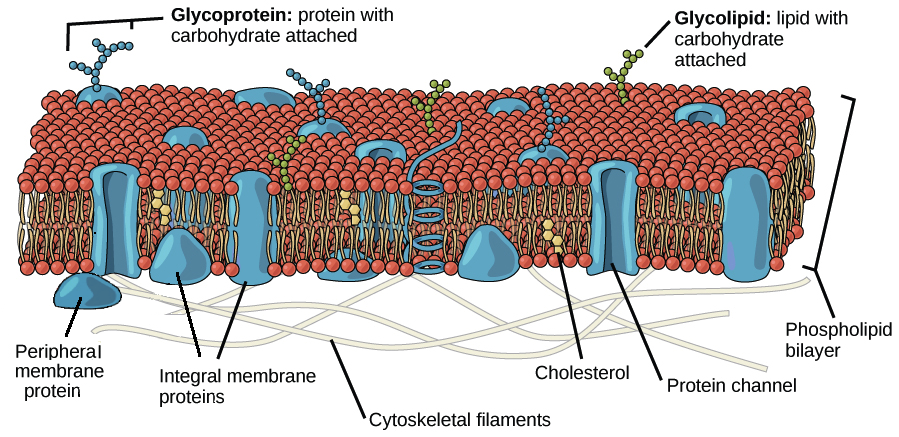
What Part Of The Membrane Do Small Uncharged Particles Diffuse Through Socratic

Lipid Bilayer Permeability Physiologyweb
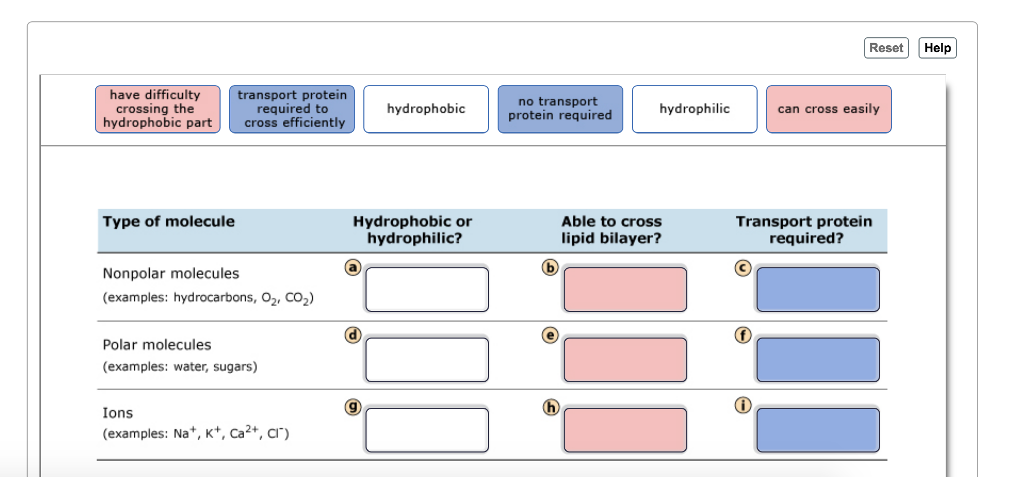
Solved Reset Help Have Difficultytransport Protein No Chegg Com
0 Response to "Are Polar Molecules Able to Cross Lipid Bilayer"
Post a Comment